Home
***We have created a new website for the List-Tsuji Group!
Please visit our new site here! This site is no longer being updated.***
 Welcome to the List Group at ICReDD, Hokkaido University!
Welcome to the List Group at ICReDD, Hokkaido University!
Catalysis can be found everywhere in human society. Our research aims to develop “perfect” chemical transformations enabled by catalysis to make human life better and more sustainable. In recent years, enantiomerically pure small organic molecules have been found as an efficient and selective catalysis, which is so-called organocatalysis. Our interest is to develop new asymmetric organocatalytic transformations through understanding and designing the microenvironment to control reactivities and selectivities. Please see the “Research topics” tab for current research topics.
News
2024.03 Haruto joined us, welcome to ICReDD!
2024.03 Ravindra has been promoted to Specially Appointed Assistant Professor. Congratulations!
2024.01 Our paper is in Nature (https://www.nature.com/articles/s41586-023-06826-7)
2023.12 Joyce joined us, welcome to ICReDD!
2023.10 Vitor and Navpreet joined the group, welcome to ICReDD!
2023.10 Prof. List visited ICReDD.
2023.04 New members joined the List group.
2023.01 Assistant Prof. Tsuji received 2023 Thieme Chemistry Journals Award!
2022.08 Prof. List visited ICReDD.
2022.04 Dr. Raut is back and Dr. Do joined the List group as postdoctoral researchers.
2021.10 Prof. List received the Nobel Prize in Chemistry! Huge congratulations!!
2020.01 The List group in ICReDD is launched!
Access
Institute for Chemical Reaction Design and Discovery (ICReDD), Hokkaido University, 05-118
Kita 21 Nishi 10, Kita-ku, Sapporo, Hokkaido, 001-0021, Japan
Research Topics
1. Asymmetric activation of inert functional groups
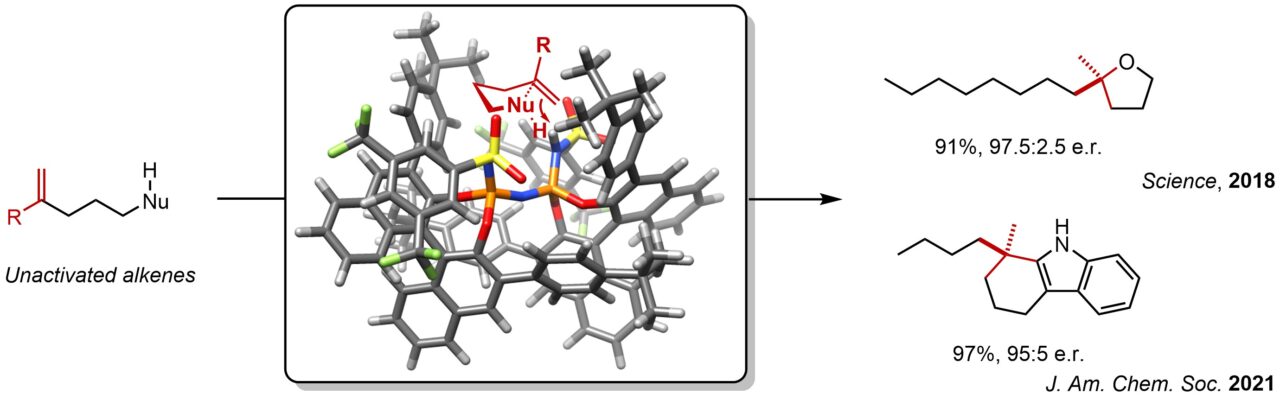
While a series of hydrocarbons are obtained from crude oil as feedstock chemicals, direct transformation of those to valuable materials is underdeveloped. For instance, alkene is an unarguably fundamental and ubiquitous functional group in chemistry, however their asymmetric hydrofunctionalizations with simple nucleophiles, such as alcohol, had remained challenging. Inspired by the extraordinary capacity of enzymes to catalyze asymmetric functionalizations of simple olefins, we designed highly acidic and confined catalysts that can promote asymmetric hydroalkoxylation reaction of simple olefins. We have also succeeded to develop highly enantioselective hydroarylation reactions, and more exiting chemistry will be revealed soon…
2. Understanding microenvironments
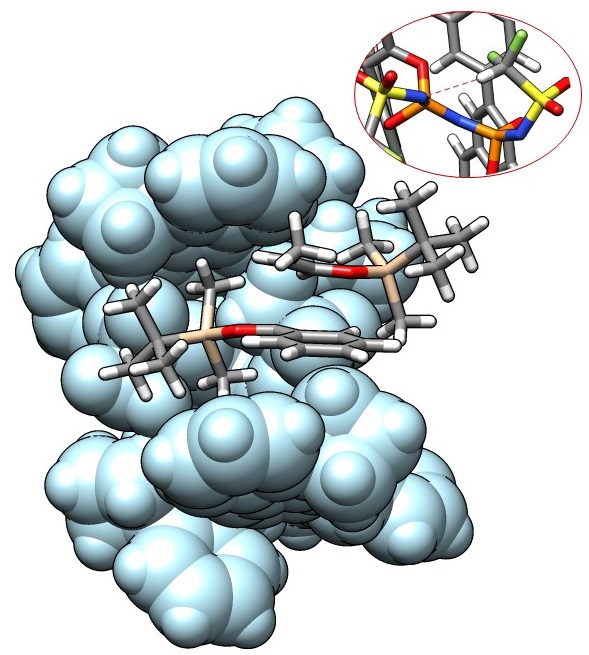
Owing to bulky substituents of our catalysts, their catalytic active sites often located in a narrow pocket; the substrates need to fit into this confined chiral microenvironment to undergo the transformation, and this is the key to achieve high enantioselectivities with simple substrates. Using quantum chemical techniques, we try to understand the origin of enantioselectivities and analyze the nature of the privileged catalysts. Recently, we have found that the key to control the IDPi-catalyzed syn– or anti-selective asymmetric Mukaiyama-aldol reactions is an unconventional C–H hydrogen bonding of the CF2H group, which controls the size of the catalytic cavity. Further evaluations of subtle electronic and steric effects are ongoing.
3. Designing highly Enantioselective Catalysts
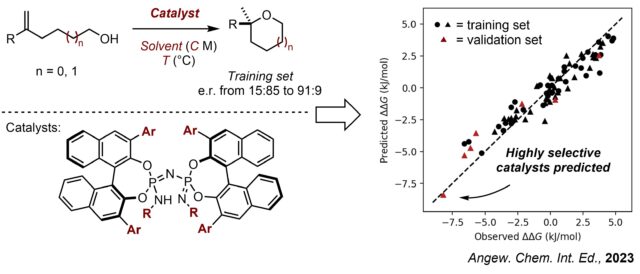
Catalyst optimization processes typically rely on the inductive and qualitative assumptions of chemists based on screening data. Although these “educated guesses” of chemists somewhat work, we aim to go beyond and rationally design more selective catalyst structures by developing methodologies to predict the enantioselectivities of catalysts. Recently, we developed a predictive model using 2D fragment descriptors, which accurately predicted highly enantioselective catalysts from training data including only moderately selective catalysts. Even more exciting chemistry is in progress.
Members
Specially Appointed Professor
- Benjamin List (Overseas PI)
Specially Appointed Associate Professor
- Nobuya Tsuji (Co-PI)
Specially Appointed Assistant Professor
- Ravindra Krushnaji Raut
Postdoctoral Researchers
- Navpreet Kaur
- Vitor Alcantara Fernandes da Silva
- Joyce Antonia Anna Grimm
Graduate Students
- D1 Satoshi Matsutani (Joint program with Maeda Group)
- M2 Hibiki Hatano (Joint program with Maeda Group)
- M2 Shuta Kataoka
- M1 Seiya Suzuki (Joint program with Maeda Group)
Undergraduate Students
- B4 Haruto Yamato
Opportunities
We welcome applications for open positions (student/postdoc) from highly motivated and talented chemists! Especially those who are willing to learn theoretical chemistry and/or chemoinformatics are highly encouraged. Please contact Assistant Professor Tsuji for details.

