A multi-institute team led by Prof. Tetsuya Taketsugu (Hokkaido University Faculty of Science, ICReDD), Prof. Taro Sekikawa (Hokkaido University Faculty of Engineering), Prof. Kenichiro Saita (Hokkaido University Faculty of Science), and Prof. Jiro Itatani (Tokyo University Institute for Solid State Physics) reported the first experimental observation verifying that a chemical reaction proceeds according to the process predicted by the long-established Woodward Hoffmann rules.
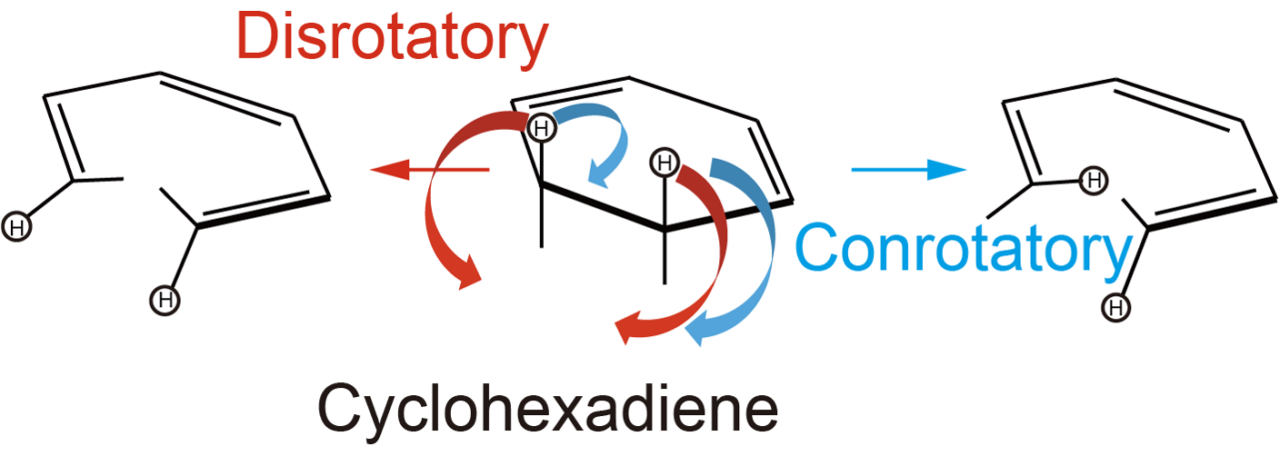
Researchers examined the ring-opening reaction of 1,3-cyclohexadiene (CHD), which can proceed two different ways depending on whether heat or light is used to activate the reaction.
When the CHD ring opens, there are two C-H bonds (see figure below) that can either both rotate in the same direction, called a conrotatory process, or they can rotate in the opposite direction, called a disrotatory process. The Woodward-Hoffmann rules provide a rationale for how the two bonds will rotate.
Depending on which way the bonds rotate relative to each other, different product molecules are formed, so predicting this rotational behavior is very important. However, since chemical reactions occur at ultrafast speeds, there has been no real-time, experimental verification of the Woodward-Hoffmann Rules.
Using state-of-the-art laser technology, the research team took femtosecond (10-15 sec) near-infrared laser pulses and converted them to soft X-ray pulses. The absorption of soft X-rays by carbon atoms in CHD is extremely sensitive to how the carbon atom is bonded to other atoms, and researchers were able to measure this absorption with femtosecond time resolution. Researchers saw a shift to higher energy absorption during the reaction, providing insight into how the bonding of the carbon atoms evolved during the reaction.
Theoretical calculations performed as part of the study showed that x-ray absorption energy is expected to shift higher for a disrotatory process and shift lower for conrotatory process. Comparing this with the experimentally observed shift to higher energy, it was verified this reaction proceeds by disrotatory process, as is predicted by the Woodward-Hoffmann rules. This is the first experimental observation verifying that a chemical reaction follows the Woodward-Hoffmann rules.