Topic Overview
Chemistry is the scientific study of the structure, properties and reactions of substances, and has been developed mainly experimentally. In recent years, however, “computational chemistry” has emerged as an approach to investigate the properties of substances and how chemical reactions occur using computers. At the beginning of the 20th century, “quantum mechanics” was established as the principle governing the behavior of atoms and molecules, and then a method to solve the Schrodinger equation approximately, which is the basic equation of quantum chemistry, was developed1 with the advent of computers. With a modern computer, the electronic structure in a molecular system can be obtained with high precision. Based on the electronic structure, we can examine the structural and energetic changes of the molecule quantitatively as the chemical reaction proceeds. Today, we can evaluate whether a chemical reaction occurs or not simply by inputting molecular structural data into a computer even if the substances or experimental equipment are unavailable.
Development at ICReDD
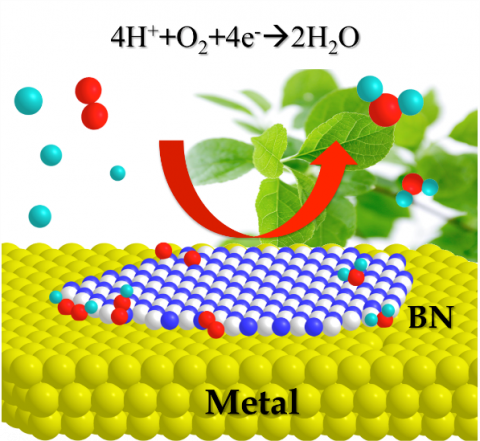
Computational chemistry has been mainly used to explain experimental results. However, it can also be used to investigate the possibility of chemical reactions even for completely unknown molecules. For example, platinum is mainly used in fuel cell catalysts but is scarce and expensive. Therefore, alternative catalyst materials are in demand. Using computational chemistry, ICReDD members proposed that a comparable catalytic activity would be generated by boron nitride (BN) supported on metal. Their research collaborators then successfully demonstrated this possibility experimentally2, which inspired world-wide research on using BN as a catalyst3. In this way, the computational, information, and experimental science teams at ICReDD work together to develop unknown molecules and functions that humanity has yet to imagine. Another example of this is that we have developed a novel synthetic method for making α,α-difluoroglycine derivatives (a bioisostere of natural glycine) based on the calculation results of the AFIR method4. Furthermore, we propose a dynamic reaction picture5,6 in which a chemical reaction proceeds while jumping from a reaction pathway to another by incorporating the effect of molecular motion into the reaction path network obtained by the AFIR method that are being developed in ICReDD. In other words, we challenge the way we view chemical reactions.
References
- 平尾公彦(監修)、武次徹也(編著)、「新版 すぐできる 量子化学計算ビギナーズマニュアル」講談社サイエンティフィク、224 pages (2015).
- Uosaki, K.; Elumalai, G.; Noguchi, H.; Masuda, T.; Lyalin, A.; Nakayama, A.; Taketsugu, T. J. Am. Chem. Soc., 2014, 136, 6542.
- Cao, Y.; Maitarad, P.; Gao, M.; Taketsugu, T.; Li, H.; Yan, T.; Shi, L.; Zhang, D. App. Catal. B: Environ., 2018, 238, 51.
- (a) Mita, T.; Harabuchi, Y.; Maeda, S. Chem. Sci. 2020, 11, 7569. (b) Hayashi, H.; Takano, H.; Katsuyama, H.; Harabuchi, Y.; Maeda, S.; Mita, T. Chem. Eur. J. 2021, 27, 10040.
- Tsutsumi, T.; Harabuchi, Y.; Ono, Y.; Maeda, S.; Taketsugu, T. Phys. Chem. Chem. Phys., 2018, 20, 1364.
- Tsutsumi, T.; Ono, Y.; Arai, Z.; Taketsugu, T. J. Chem. Theory Comput., 2018, 14, 4263.

