Topic Overview
Hydrogel is a material in which a large amount of water is confined within the three-dimensional network structure of a chain-like molecule called a “polymer”. Because hydrogels have both solid and liquid properties, they are unique in that they are self-standing like solid but are permeable to substances like liquid, and solution chemical reactions inside the hydrogel also can be performed. Since biological tissues also contain water, hydrogels are expected to be biocompatible which allows us to use hydrogels inside of living organisms. However, hydrogels in general are very fragile, like jelly, and are not suitable for use as materials. To solve this problem, we have developed a tough double-network hydrogel (DN gel) by combining brittle and stretchable networks1,2. The DN gel contains about 90% water but is extremely tough and does not break even when run over by a truck. Utilizing this strength and properties as a gel, we are conducting a variety of application research of DN gels.
Development at ICReDD
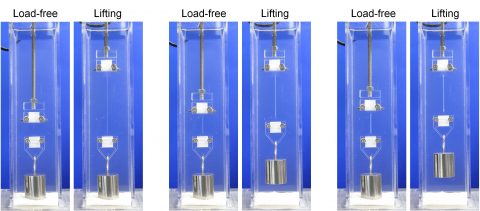
We are pursuing to develop new fields in ICReDD through the mutual inspiration of DN gel fabrication, chemical reaction design, and biological tissues. For example, we are developing new chemical reactions using DN gel as a special environment for reaction, and are trying to further enhance the functionality of DN gels brought about by the new chemical reactions such as the development of DN gels that get stronger and larger through physical training like muscles3. In terms of interaction with living tissue, we aim to develop new medical materials based on controlling the cross-interaction between the DN gel and a living body, such as the discovery that the behavior of cells cultured on the surface of a DN gel varies greatly depending on the surface properties (potential and flexibility) of the gel in ICReDD4.

References
- Gong, J. P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Adv. Mater. 2003, 15, 1155.
- Gong, J. P. Soft Matter. 2010, 6, 2583.
- Matsuda, T.; Kawakami, R.; Namba, R.; Nakajima, T.; Gong, J. P. Science 2019, 363, 504.
- Frauenlob, M.; King, D. R.; Guo, H.; Ishihara, S.; Tsuda, M.; Kurokawa, T.; Haga, H.; Tanaka, S.; Gong, J. P. Macromolecules 2019, 52, 6704.

