Carboxylic acids are ubiquitous motifs with appearance in compounds ranging from pharmaceuticals to agrochemicals. As such, derivatization of these valuable synthons has been a highly sought-after goal for the generation of molecular complexity and functions. A classical transformation involves the functionalization of the α-position of carboxylic acids through enolate intermediates. In comparison, functionalization at a more distal β-position has seen much less development. In both scenarios, “protection” of the acid proton by conversion to ester or amide is often necessary, as its acidity can compete with the production of desired enolates. Strategies allowing for the direct functionalization of carboxylic acids would thus be highly desirable to avoid multi-step functional group interconversions.
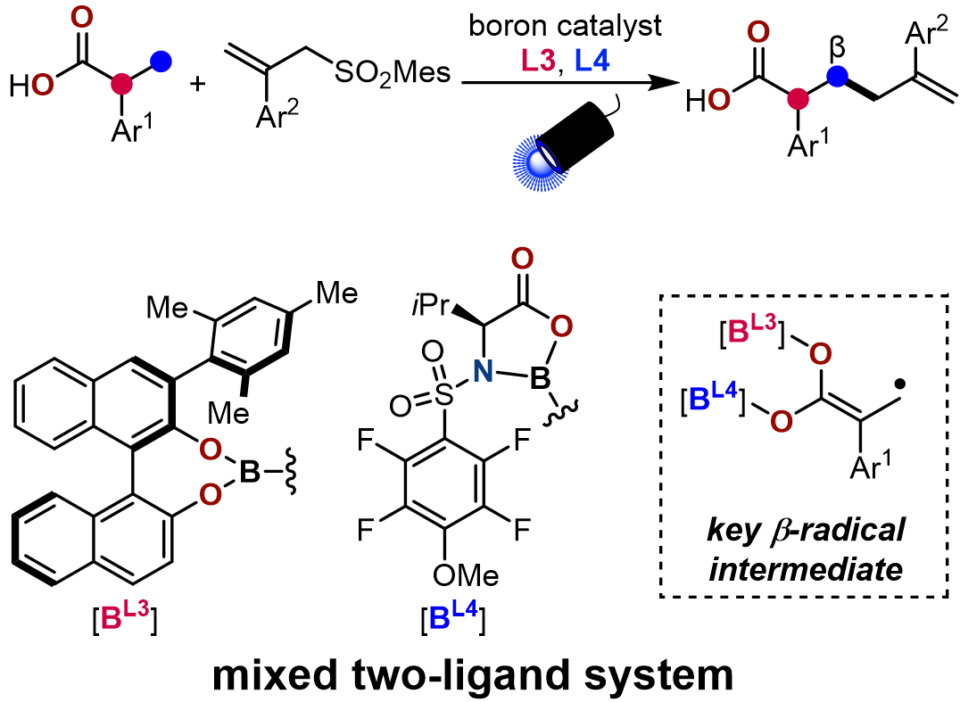
Previously, the Sawamura group reported a boron-catalyzed direct α-allylation of carboxylic acids. Through the formation of a diboron enediolate in combination with a bipyrenol ligand, photoinduced electron transfer with allyl sulfones can be achieved, and subsequent radical-radical coupling is proposed to afford the α-allylated products. We hypothesized that a further deprotonation of the diboron enediolate-derived radical cation intermediate would transpose the α-radical to the β-position, allowing for the switch to β-allylation pathway . In this work, we discovered that this goal can be accomplished by a dual-ligand system consisting of a BINOL-type ligand and an amino-acid derived ligand. A wide collection of carboxylic acid, including bioactive molecules, can be β-allylated with excellent selectivity. Taking advantage of the remaining carboxylic acid handle, further derivatization of these products was also demonstrated to rapidly increase the molecular complexity. Mechanistic studies first established the critical role of β-deprotonation in the catalytic cycle. Theoretical calculations suggested that the dual ligands promote β-allylation through electronically favoring β-deprotonation while sterically hampering the competing α-allylation. This methodology is expected to not only provide avenues for novel carboxylic acid compounds but also introduce new concepts in synergistic catalysis.