New mechanochemical method for synthesizing organosodium reagents has promise to reduce reliance on lithium and improve sustainability in general organic synthesis.

Highly reactive organometallic reagents, like organolithiums (molecules with a carbon–lithium bond) are essential reagents in organic synthesis because of their applications from polymer synthesis to pharmaceuticals, and more. Lithium resources, however, are difficult to access because concentrated deposits are geographically restricted and modern extraction methods are burdened with environmental costs. Replacing lithium with sodium would be a significant contribution towards environmentally friendly organic synthesis because it is >1000 times more abundant and its extraction from seawater is sustainable and inexpensive.
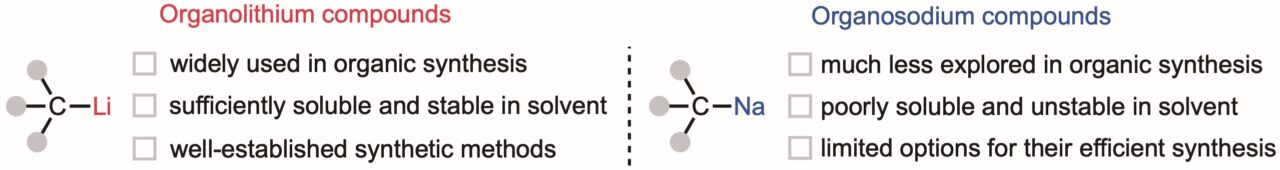
Despite sodium metal being widely available and organosodium being highly reactive, the conventional method for synthesizing organosodium reagents requires highly toxic reagents and involves complex experimental procedures. Moreover, the use of organosodium reagents is paradoxically limited because they react with nearly all solvents except alkanes (e.g. hexanes C6H14), in which they have low solubility. Recently, however, a joint research team with researchers from WPI-ICReDD at Hokkaido University, Newcastle University, and University of Birmingham may have developed the first environmentally friendly organosodium methodology that could sustainably replace lithium in modern day organic synthesis. These findings were published in Nature Synthesis on December 8, 2025.
The new strategy uses ball-milling mechanochemistry techniques which have recently demonstrated great success in facilitating organic reactions in the solid state with little to no liquid additives. Their experiments demonstrated that organosodium reagents can be generated within 5 minutes by reacting organic halides with lumps of sodium metal in a ball mill in the presence of a small amount of hexanes as a liquid additive. The obtained organosodium reagents were successfully used in reactions with various reactants under the same mechanochemical conditions, producing versatile new compounds in high yield.
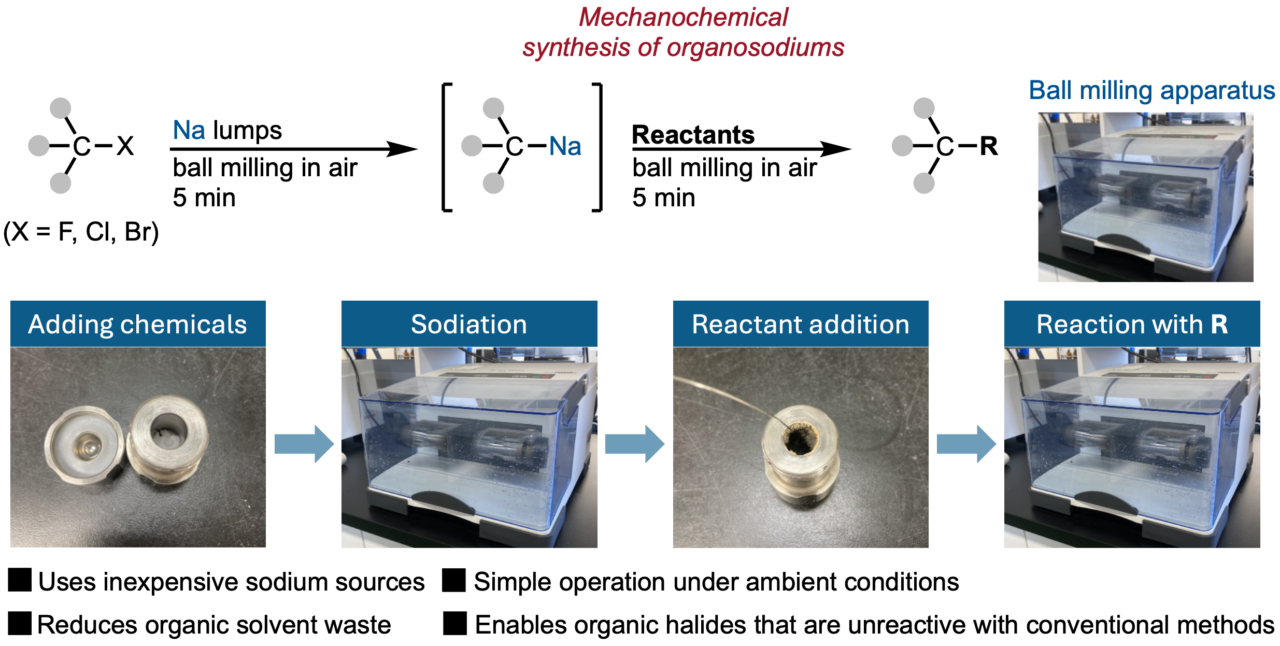
When dissolved in solution, organosodium reagents will decompose rapidly from trace amounts of water or oxygen. However, the mechanochemical reactions proceed in the solid-state without concern towards presence of air and moisture, a quality distinct from conventional solvent-based methods that require rigorously dried solvent and inert gas. In addition, their mechanochemical method successfully transformed poorly soluble organic halides and organic fluorides that are unreactive under conventional methods, significantly expanding the applicability of organosodium in organic synthesis. This study establishes a simple, efficient, and more environmentally friendly approach for the synthesis of organosodium reagents. With these findings serving as a new foundation, accelerated developments for organosodium applications in organic synthesis are anticipated.

Original article:
Keisuke Kondo, et al. Mechanochemical synthesis of organosodium compounds through direct sodiation of organic halides. Nature Synthesis. December 8, 2025.
DOI: 10.1038/s44160-025-00949-7
Funding:
This work was supported by the Japan Society for the Promotion of Science (JSPS) via KAKENHI grants 22H00318, 24H00453, 24H01050, 24H01832, and 22K18333, by the JST via CREST grant JPMJCR19R1 and FOREST grant JPMJFR201I, and by the Institute for Chemical Reaction Design and Discovery (ICReDD), which was established by the World Premier International Research Initiative (WPI), MEXT, Japan. The authors thank the Leverhulme Trust for generous financial support via Research Grants RPG-2022-231 and RPG-2023-159.
Contacts:
Professor Hajime Ito
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD)
Hokkaido University
Tel: +81-11-706-6561
Email: hajito[at]eng.hokudai.ac.jp
Associate Professor Koji Kubota
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD)
Hokkaido University
Tel: +81-11-706-8127
Email: kbt[at]eng.hokudai.ac.jp
Assistant Professor Samuel Jacob
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD)
Hokkaido University
Tel: +81-11-706-9667
E-mail: public_relations[at]icredd.hokudai.ac.jp
Naoki Namba
Public Relations & Communications Division
Office of Public Relations and Social Collaboration
Hokkaido University
Tel: +81-11-706-2185
Email: en-press[at]general.hokudai.ac.jp

