
Researchers from ICReDD recently reported the use of cyclic polyketone “hosts” to bind alkali metal ion “guests”. This binding phenomenon enabled cyclic polyketones to function as catalysts for a halogen exchange reaction. Finding new molecules that can act as ion traps is important for catalysis, as well as applications that require ion adsorption or transportation.
The Inokuma group in ICReDD used cyclic polyketones with 1,3-diketone repeating units to bind alkali metal ions, and they found the binding ability was dependent on the ring size of the molecule. X-ray and computational analysis found that the ion trapping occurred via coordination bonds between the alkali metal ion and multiple carbonyl oxygen atoms in the cyclic polyketone.
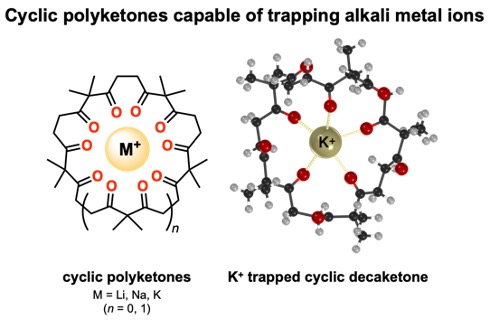
Using this binding ability, it was possible to promote a halogen exchange reaction (Finkelstein reaction) despite using low polarity solvents. The catalytic ability of the cyclic polyketone for this reaction was proportional its ion binding strength.Additionally, researchers found that modifying both ends of linear polyketones also resulted in an improved ability to bind alkali metal ions. Based on these findings, the selective trapping of alkali metal ions can be achieved by precise control of the polyketone compounds.
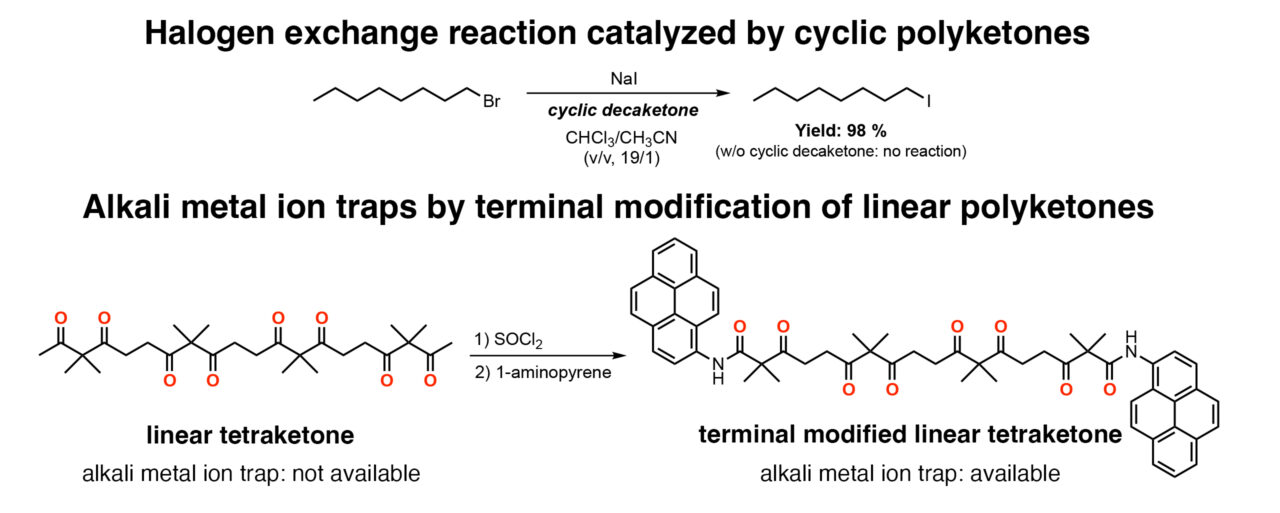
The generation of cyclic compounds capable of selectively trapping target ions expands the possibilities for the development of polyketone-based catalysts and functional molecular materials.
Original paper
Alkali Metal Ion Binding using Cyclic Polyketones
Narito Ozawa, Kilingaru I. Shivakumar, Muthuchamy Murugavel, Yuya Inaba, Tomoki Yoneda, Yuki Ide, Jenny Pirillo, Yuh Hijikata, Yasuhide Inokuma
Chem. Commun., 2021, 58, 2971–2974. (Inside front cover) DOI: 10.1039/d2cc00361a
Selected for the themed collection: Chemical Communications HOT Articles 2022 and 2022 Pioneering Investigators

