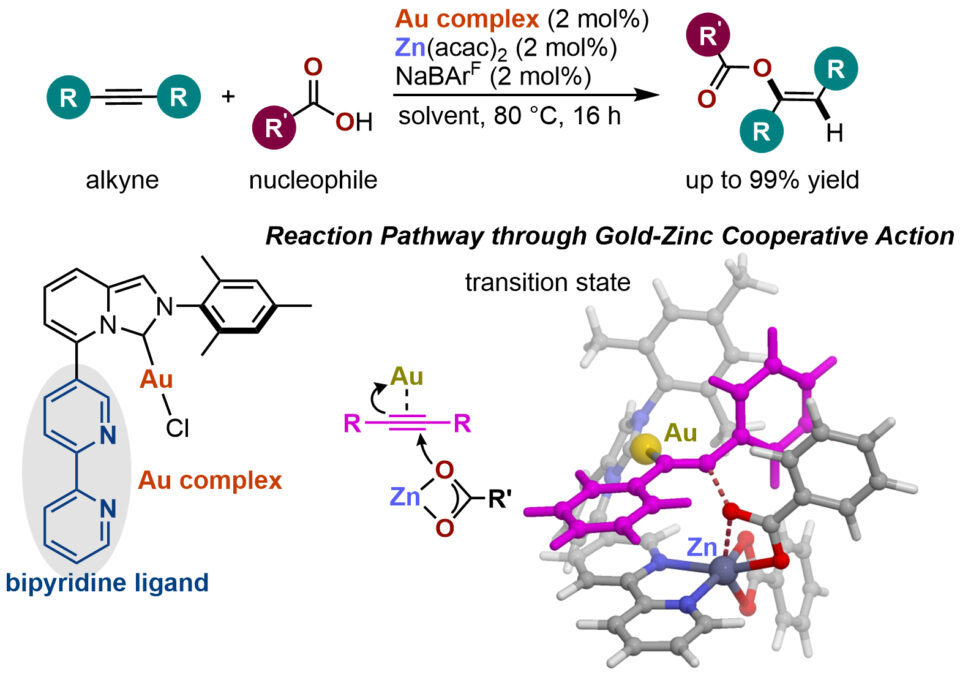
For intermolecular reactions that form a bond between two different molecules, it is important to activate both molecules so they will react readily with each other. Each starting material may require a different activation method, so by utilizing a catalyst with two different metal atoms, it becomes possible to activate both starting materials with a single catalyst.
PhD student Vishal Rawat and Specially Appointed Assistant Professor Kosuke Higashida from the lab of ICReDD PI Masaya Sawamura utilized this idea to create a bimetallic catalyst containing gold and zinc, which enabled the efficient addition of weak nucleophiles such as carboxylic acids or phenols to unactivated molecules containing triple bonds. First, researchers created a gold complex with a bipyridine ligand attached. The bypridine can capture transition metal ions to create a catalyst containing two metal ions. Many transition metals were tested, and it was found that pairing zinc, which is good at activating nucleophiles, with gold, which is good at activated alkynes, resulted in a catalyst that produced the highest yields for the nucleophilic addition reactions being studied. Reaction path calculations also supported the idea that cooperative interactions between the gold and zinc promote this reaction.
This work provides a viable strategy for designing highly efficient catalysts that incorporate two different metal ions.