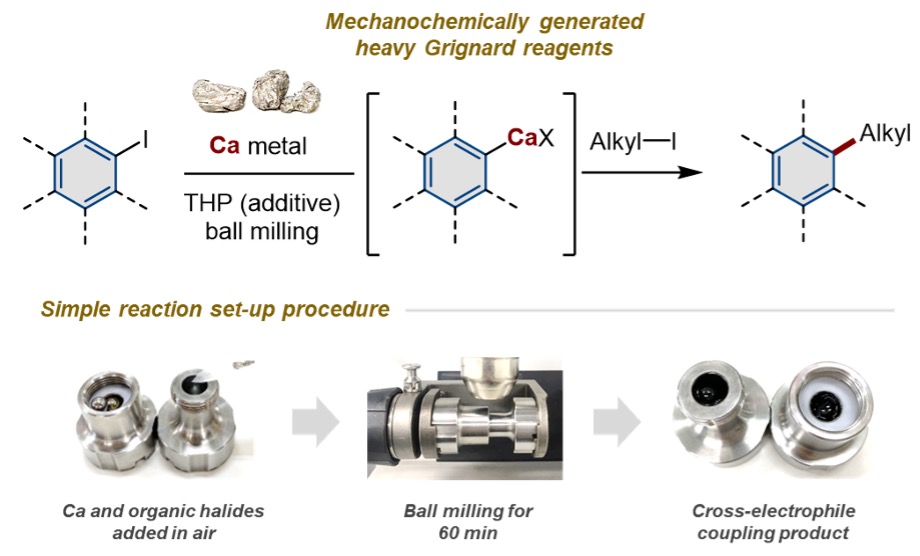
Grignard reagents are one of the most widely used classes of chemical compounds in the chemical industry. They enable the formation of carbon-carbon bonds, an essential building block for organic molecules. Typically, these reagents are formed by mixing magnesium metal with organohalide compounds. By using calcium instead of magnesium, so-called “heavy Grignard reagents” can be made to explore the novel reactivity that results from the comparatively higher polarity of calcium-carbon bonds. However, the study of heavy Grignard reagents is impeded by the low reactivity of calcium metal, which requires a laborious pre-activation step that involves toxic ammonia or harsh reaction conditions.
Researchers at ICReDD have addressed this issue by developing a streamlined way to make heavy Grignard reagents without the need for a pre-activation step. In the mechanochemical method developed here, an organohalide and commercially available calcium are put inside a ball mill with a small amount of organic solvent additive. The mechanical impact of the ball provides enough energy to activate the calcium without the need for harsh conditions, dry organic solvents, or strict temperature control. With this operationally simple method in hand, researchers successfully demonstrated the first example of the alkylation of arylcalcium halides with alkyl electrophiles. Importantly, the use of calcium instead of magnesium for this reaction resulted in a significantly higher yield of the desired product.
This work demonstrates the promise of calcium-based Grignard reagents, showing they can result in novel reactivity and improved yield. Additionally, the mechanochemical protocol reported here provides researchers with a simple, non-toxic method for creating heavy Grignard reagents, opening the door for further investigation into this largely unexplored field.