The MANABIYA Program enables researchers from other institutes, universities and companies to come to ICReDD for a short-term period of training and joint research. The following paper includes contributions that were made possible by the participation of lead author Shunsaku Yasumura in the MANABIYA System. He worked with Prof. Tetsuya Taketsugu at ICReDD for ten weeks in 2021, where he learned the artificial force induced reaction (AFIR) method. During his time in MANABIYA, Shunsaku was a PhD student in Graduate School of Chemical Sciences and Engineering, Hokkaido University, in the lab of Professor Ken-ichi Shimizu. He is currently an assistant professor at the Institute of Industrial Science, The University of Tokyo.
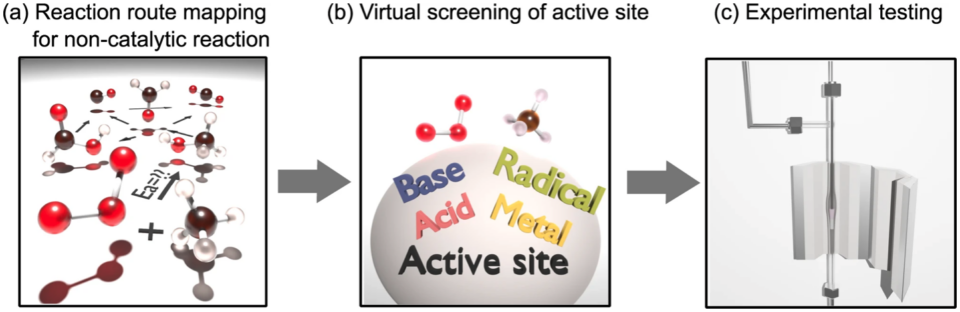
Natural gas has become a widely used fuel, but the resulting unburned methane waste is an issue, as methane has a greenhouse gas effect 22 times greater than that of carbon dioxide. The catalytic combustion of methane is becoming a promising strategy for dealing with these emissions. However, with the conventional method that uses oxygen molecules as the oxidizer, it was not possible to achieve low temperature (below 200°C) combustion of methane. This study utilized automated reaction route mapping via the Artificial Force Induced Reaction (AFIR) method to guide the design of more efficient and robust main group element catalysts for the combustion of methane with ozone.
Researchers first surveyed different possible catalyst types by choosing model molecules to represent each active site type (redox, radical, Brønsted acid, Brønsted and Lewis base) and using AFIR to compute reaction routes for the combustion of methane at these sites. Sulfuric acid, the model molecule for a Brønsted acid site, was the most promising, yielding an activation energy lower than the uncatalyzed process. Researchers then used AFIR to evaluate catalysts with differing acidities and found a general trend that more strongly acidic catalysts exhibit more catalytic activity. Based on this trend, an Hß-Zeolite catalyst, which is highly acidic, was tested experimentally and was found to be capable of low-temperature (low activation energy) methane combustion. The Hß-Zeolite catalyst exhibited a reaction rate 442 times higher than the conventional palladium-based catalyst, Pd5Al2O3. While this conventional catalyst is subject to active site poisoning in the presence of H20 and SO2, the Hß-zeolite catalyst exhibited resistance to H20 and SO2, successfully combusting 100% of the methane during endurance testing while exposed to to water and sulfur oxide.
This study demonstrates the usefulness of automated reaction route mapping as a strategy for computationally designing catalysts.