Catalysts are important substances that promote chemical reactions. They have been used to develop new medicines and reduce pollutants in car exhaust and they play an integral role in achieving a carbon neutral society. An important class of catalysts consists of metal nanoparticles dispersed on a supporting material. However, it has been unclear how exactly reactions proceed on such catalysts, including how reactants attach and migrate along the support surface, and how such dynamic behavior may affect the reaction.
ICReDD’s Junior PI (Associate Professor) Min Gao collaborated with a multi-institute team led by Professor Satoru Takakusagi of Hokkaido University’s Institute for Catalysis on a study recently published in The Journal of the American Chemical Society that unravels the details of the diffusion and reaction of methoxy intermediates during methanol decomposition on a TiO2 (110) surface with dispersed platinum nanoparticles.
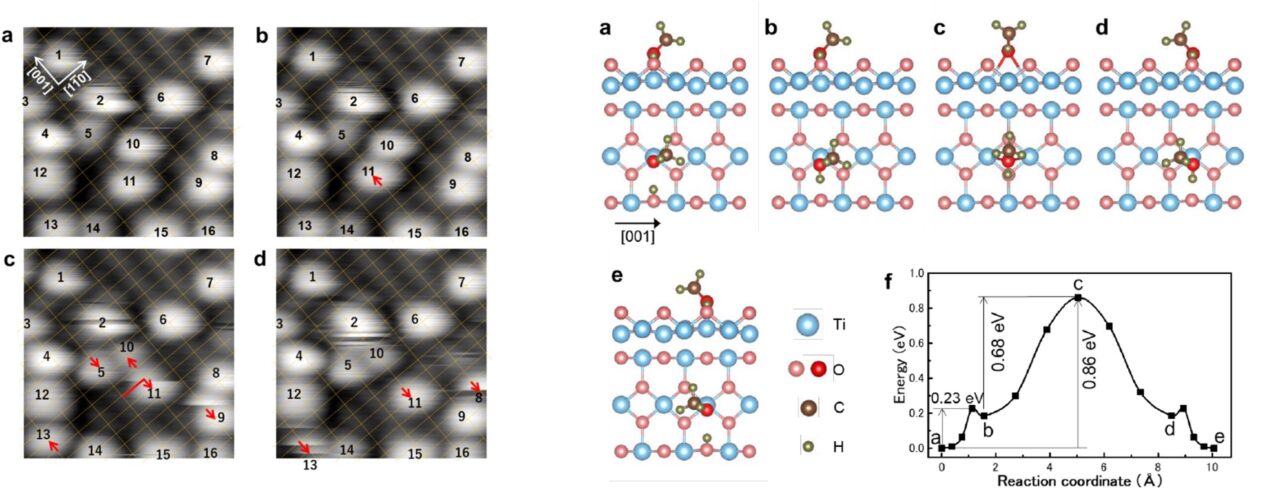
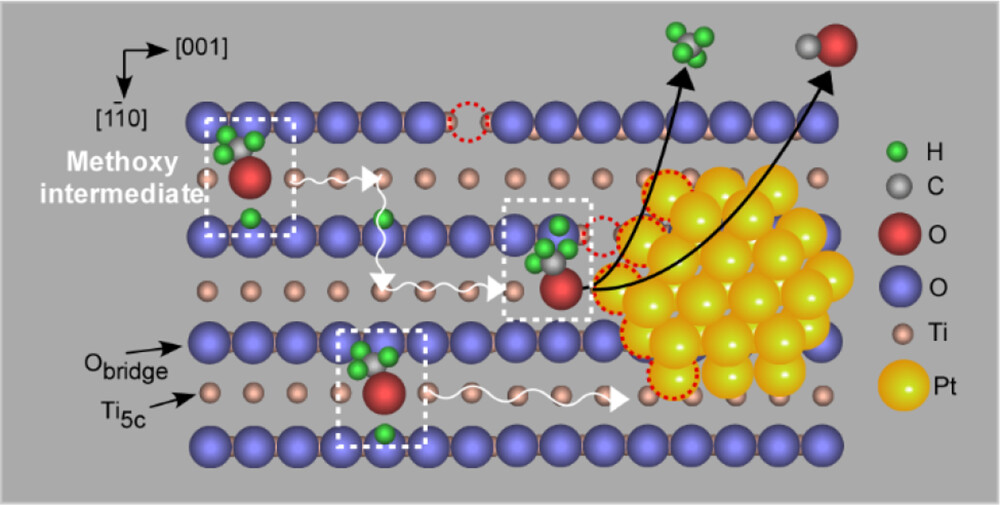
Using scanning tunneling microscopy (STM) and theoretical simulations, the team analyzed three elementary steps in the reaction process: methanol adsorption, diffusion, and decomposition. Methanol was found to dissociate upon adsorption on a Pt nanoparticle. The resulting methoxy intermediates spillover to the TiO2surface where it was observed via STM that they diffuse along two specific directions. The methoxy species then arrives again at a Pt nanoparticle and undergoes decomposition. Computer simulations using density functional theory verified the plausibility of the methoxy diffusion along the TiO2 surface and proposed mechanisms for how the methanol diffuses in each direction.
Researchers then found that the temperature of methanol decomposition and the resulting ratio of products depended on the density of Pt nanoparticles on the TiO2 surface. A higher Pt density resulted in decomposition occurring at lower temperatures, indicating a higher reactivity and providing evidence that decomposition primarily occurs at Pt nanoparticles rather than on the TiO2 support surface. The relative ratio of products also depended on Pt density, and was attributed to methoxy intermediates primarily reacting at interfacial Pt-TiO2sites, rather than on flat terraces of Pt.
This study provides atomic-level understanding of how reactions proceed on metal supported catalysts and gives key insight into the factors that influence catalytic activity and product selectivity.