γ-Lactones are structural motifs widely present in natural organic compounds and pharmaceuticals, and various synthetic methods have been developed for their construction. Now, researchers at ICReDD have developed a method that uses light irradiation to enable the building of γ-lactones from inexpensive formate salts and allylic alcohols, both of which are very simple starting materials. This is the first reported one-step synthesis of γ-lactones from allylic alcohols, and the reaction was successful for a variety of differently substituted allylic alcohols.
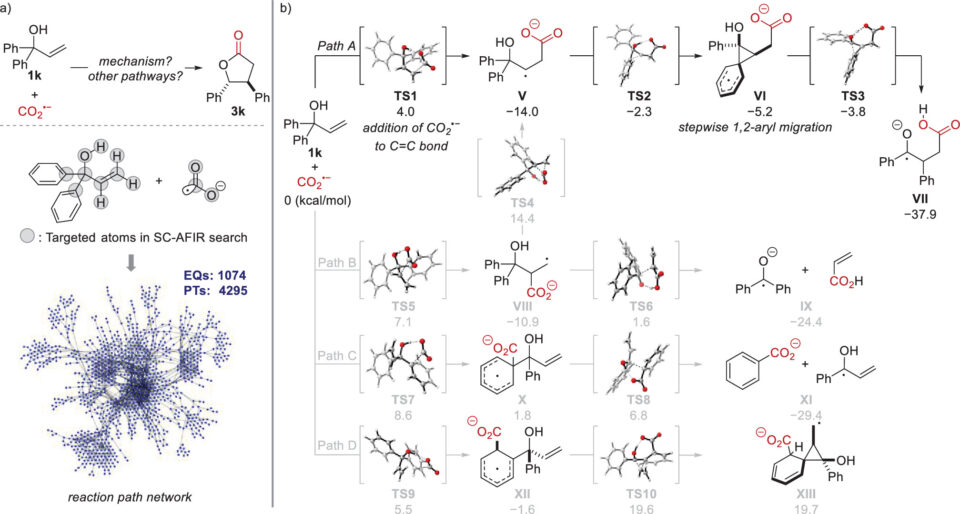
It was found that using an allylic alcohol with two aryl groups bonded to the same carbon next to the oxygen atom resulted in the transfer of one of the aryl groups to the neighboring carbon atom during the reaction. To understand the mechanism of this novel rearrangement, researchers used the artificial force induced reaction (AFIR) method to perform a comprehensive, computational reaction path search, identifying all possible reaction pathways from a single starting molecular structure. The pathway of the main reaction involving the aryl group rearrangement was found to be both kinetically and thermodynamically favorable compared to other side reactions, thus clarifying the overall reaction mechanism.
This article is open access and can be viewed by everyone.
For details, please see the original article here:
Saeesh R. Mangaonkar, Hiroki Hayashi, Wataru Kanna, Suvankar Debbarma, Yu Harabuchi, Satoshi Maeda, and Tsuyoshi Mita. DOI: 10.1021/prechem.3c00117