1,2-Bis(diphenylphosphino)ethane (DPPE), a representative of diphosphine ligands, forms complexes with various transition metals as a chelating bidentate ligand, exhibiting catalytic and luminescent functions. Meanwhile, diphosphine ligands with rigid linear or bent structures are important compounds for constructing supramolecular frameworks. Additionally, unsymmetric diphosphine ligands with different substituents on each of the phosphorus atoms allow precise control over the electronic and steric properties of metal complexes by selecting appropriate substituents, paving the way for the creation of new highly active metal catalysts and highly functional supramolecules.
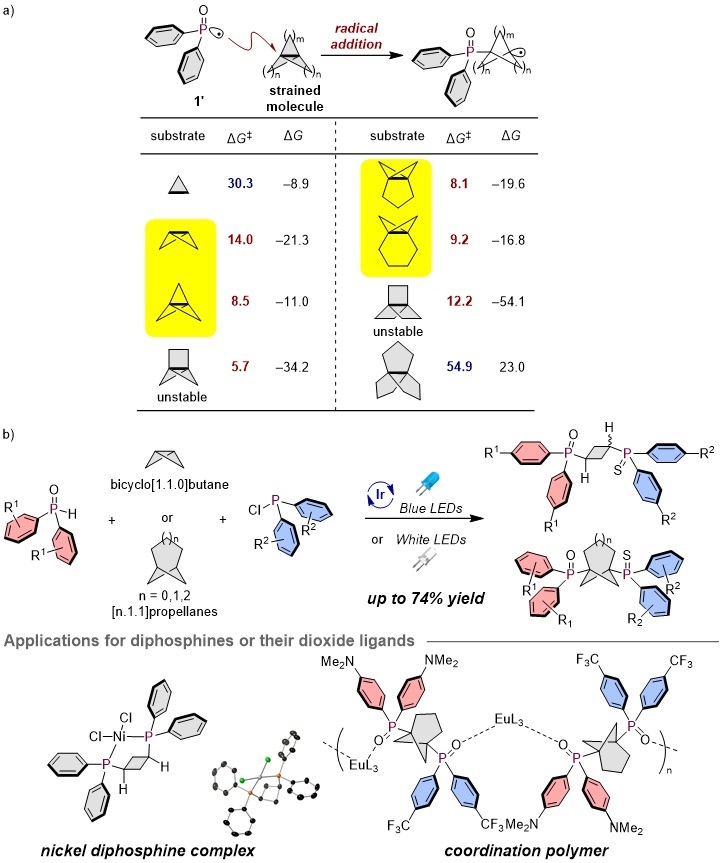
In this study, the research group led by Mita and co-workers has established a simple synthetic method for symmetric and unsymmetric phosphorus ligands derived from the radical diphosphination of strained small ring compounds such as bicyclo[1.1.0]butane and [n.1.1]propellanes. This has led to the synthesis of new transition metal catalysts and coordination polymers. Notably, it was revealed that the diphosphine complexes with a cyclobutane skeleton derived from bicyclo[1.1.0]butane coordinate bidentately to nickel. Additionally, the diphosphine dioxide ligands synthesized from [3.1.1]propellane could be induced into bent-structured coordination polymers.
During the development of this reaction, the activation energy for the radical addition of the initial phosphoryl radical was efficiently determined using quantum chemical calculations with the AFIR method, facilitating the reaction development. Highlighted compounds in yellow were examined due to their stabilities.
This article is open access and can be viewed by everyone.
For details, please see the original article here:
Chandu G. Krishnan, Hideaki Takano, Hitomi Katsuyama, Wataru Kanna, Hiroki Hayashi, and Tsuyoshi Mita. DOI: 10.1021/jacsau.4c00347

