The MANABIYA Program enables researchers from other institutes, universities, and companies to come to ICReDD for a short-term period of training and joint research. The following paper includes contributions that were made possible by the participation of corresponding author Lecturer Hiroki Shigehisa (Faculty of Pharmacy, Musashino University) in the MANABIYA Program in 2023. He worked with Prof. Satoshi Maeda and Prof. Tsuyoshi Mita at ICReDD and learned the artificial force induced reaction (AFIR) method.
Recently, metal-catalyzed hydrogen atom transfer (MHAT) has gained attention in the field of organic synthesis. MHAT is a reaction that utilizes a metal catalyst to transfer a hydrogen atom, generating a radical intermediate. This radical intermediate is highly reactive, making it effective for developing synthetic methods that were previously challenging. One of the key features of MHAT is that it proceeds under mild reaction conditions, making it an essential tool in the construction of complex molecules. In 2013, Shigehisa at Musashino university developed a novel method combining MHAT with radical-polar crossover (RPC) using a cobalt catalyst. This method enables the synthesis of more diverse and complex molecular structures by transitioning from a radical reaction pathway to an ionic one. This approach has since been applied by chemists worldwide.
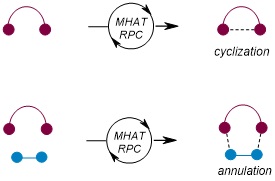
In this research, we focused on the synthesis of heterocycles, which are skeletons commonly found in pharmaceuticals. Previously, constructing heterocycles via the MHAT/RPC method was achieved through “cyclization: by single bond connection” (Figure 1). However, this study revealed that this method could be extended to “annulation: by two bond connections” significantly expanding the scope of heterocycle synthesis. This method is highly effective for constructing complex molecular structures and opens new possibilities for the synthesis of pharmaceuticals and functional materials.
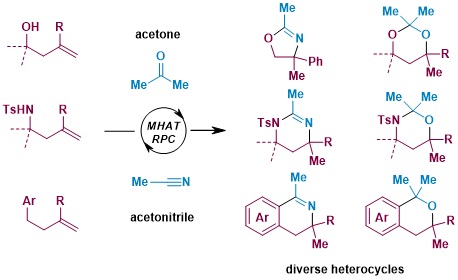
One noteworthy point is the expanded range of applicability based on the choice of starting materials and reaction solvents. In this study, we found that by using acetone, a common reaction solvent, it not only serves to dissolve the reactants but also plays a role as a structural component in forming unique ring structures (Figure 2). It was also confirmed that by using other solvents such as acetonitrile, a wide variety of molecular structures could be synthesized.
Furthermore, to deepen our understanding of the reaction mechanism, we employed a specialized computational technique called the AFIR method (Artificial Force Induced Reaction). AFIR is a type of quantum chemical calculation that simulates how molecules react, predicting energy changes and reaction mechanisms. By using this technology, we could predict which intermediates form temporarily during the reaction and identify the key stages of the reaction, leading to a deeper understanding when combined with experimental data. In this study, we gained insights into how a “cationic alkyl cobalt complex” intermediate, formed during the reaction, affects product types and selectivity.
By combining experimental results with cutting-edge computational techniques, we have established a new synthetic method that holds promise for future applications in drug development and materials science. This could accelerate the development of innovative drugs and new materials.
For details, please see the original article here:
Takuma Sugimura, Ren Yamada, Wataru Kanna, Tsuyoshi Mita, Satoshi Maeda, Bartłomiej Szarłan, Hiroki Shigehisa. DOI: 10.1021/acscatal.4c05195

