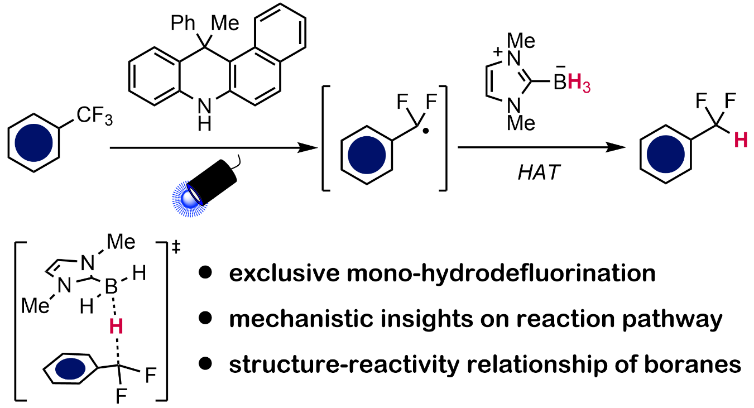
Organofluorine compounds play a crucial role in functional molecules with broad applications ranging from pharmaceutical to materials science. Trifluoromethyl groups (CF3) represent a privileged motif, widely utilized due to their distinctive molecular properties. In recent years, there has been growing interest in difluoromethylene (CF2) groups as a versatile building block to expand chemical space and identify novel bioactive compounds. One strategy to synthesize these motifs is through selective defluorination of the readily available CF3 groups. These methods can however be hampered by high C‒F bond strengths as well as risk of overdefluorination. Methods to circumvent these issues often rely on photochemical or electrochemical activation. As one prominent example, difluoromethyl (CF2H) moieties can be prepared from CF3 groups via hydrodefluorination reactions.
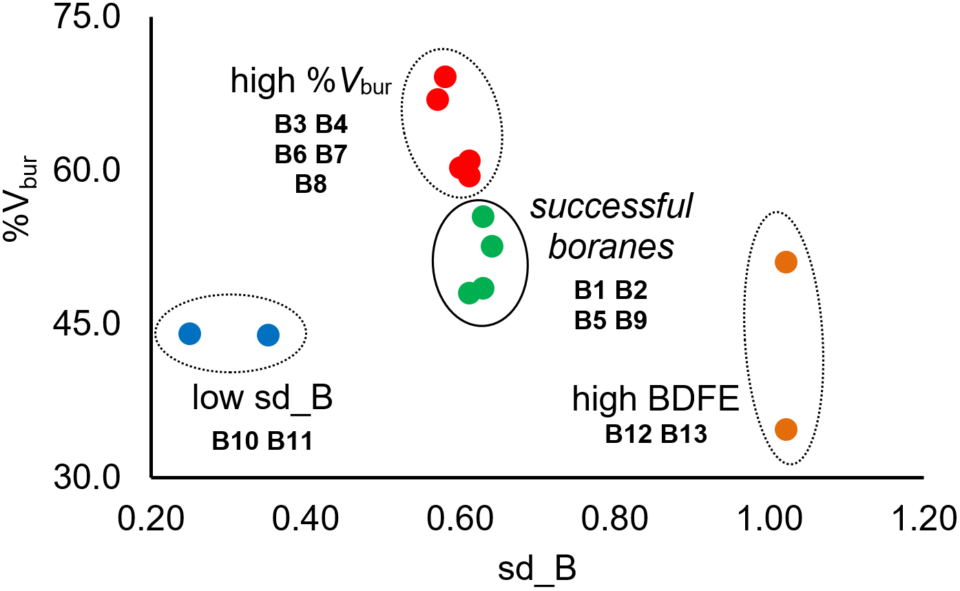
In this work, we report our mechanistic investigations on selective hydrodefluorination of trifluoromethylarenes (ArCF3) using N-heterocyclic carbene boranes (NHC‒BH3) as the hydrogen atom transfer reagent. Hydrogen atom transfer, or HAT, is a fundamental mechanism in radical reactions. Photoexcitation of a recently reported photocatalyst under blue LED irradiation enables the selective reduction of ArCF3 to generate ArCF2• radical species. Subsequent HAT between NHC‒BH3 and this radical intermediate affords the desired product. Through detailed experimental and computational studies, we were able to disapprove of an alternative mechanism and provide support for our proposed pathway. Importantly, computation and data science approaches allowed us to build a classification model based on easily obtainable molecular descriptors to explain the reactivity differences across a selection of boranes. Our synthetic method is applicable to ArCF3 containing attractive motifs such as amino acids and carbohydrates, thus giving access to new CF2H-containing compounds. Overall, this study provides in-depth mechanistic and quantitative understandings of boryl radical chemistry with relevance to hydrodefluorination reactions.
For details, please see the original article here:
Amit K. Jaiswal, Bastian Bjerkem Skjelstad, Satoshi Maeda* and Dennis Chung-Yang Huang*