Researchers have shown mechanical force can start chemical reactions, making them cheaper, more broadly applicable, and more environmentally friendly than conventional methods.
Chemical reactions are most conventionally prompted by heating up the reaction mixtures. Within the last ten years, there has been extensive research on “photoredox catalysts” that can be activated by visible light and enable highly specific and efficient chemical reactions. However, these reactions often require a large amount of harmful organic solvents, making them applicable only to soluble reactants.
“Piezoelectric materials” such as barium titanate are known to generate electric potentials when a mechanical pressure is applied to them, which is why they are used in microphones and lighters. In the current study published in Science, the research team led by Hajime Ito and Koji Kubota of the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) at Hokkaido University proved this electric potential can also be used to activate chemical reactions. “In our system, we use the mechanical force provided by a ball mill to activate a piezoelectric material for redox reactions, while eliminating the use of organic solvent,” says Koji Kubota. They call it a mechanoredox reaction as opposed to a photoredox reaction.
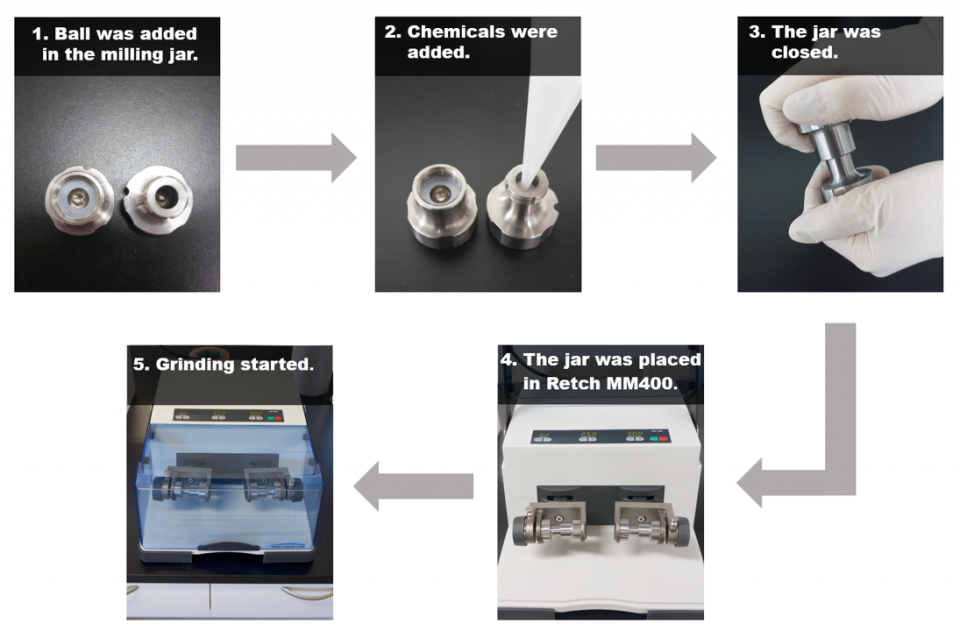
The team demonstrated that electric potentials derived from piezoelectric material (BaTiO3) activate a compound called aryl diazonium salts generating highly reactive radicals. The radicals undergo bond-forming reactions such as arylation and borylation reactions — both of which are important in synthetic chemistry — with high efficiency. The team also showed that the borylation reaction could occur by striking the mixture in a plastic bag with a hammer.

“This is the first example of arylation and borylation reactions using mechanically induced piezoelectricity,” says Koji Kubota. “Our solvent-free system using a ball mill has enabled us to eliminate organic solvents, making the reactions easier to handle, more environmentally friendly, and applicable even to reactants that cannot be dissolved in the reaction solvent.” They could also recycle the barium titanate and achieve better yields than photoredox reactions, even further increasing the attractiveness of this approach.
“We are now exploring the tunability of the mechanically generated electric potential. Together with computational predictions, we aim to extend the applicability of this technique,” says Hajime Ito. “Our goal is to complement or at least partly replace existing photoredox approaches and provide an environmentally friendly and cost-efficient alternative to be used in industrial organic synthesis.”

Original article:
Kubota K., Pang Y., Miura A., Ito H. Redox Reactions of Small Organic Molecules Using Ball Milling and Piezoelectric Materials. Science, December 19, 2019.
DOI: 10.1126/science.aay8224
Funding:
This study was supported by the Japan Society for the Promotion of Science (JSPS)’s KAKENHI grants (JP18H03907, JP17H06370, JP19K15547), and the Japan Science and Technology Agency (JST)’s CREST grant (JPMJCR19R1). The Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) was established by the World Premier International Research Center Initiative (WPI), MEXT, Japan.
Contacts:
Professor Hajime Ito
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD)
Hokkaido University
Email: hajito[at]eng.hokudai.ac.jp
https://www.icredd.hokudai.ac.jp/ito-hajime
Naoki Namba (Media Officer)
Institute for International Collaboration
Daniel Schenz (Media Officer)
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD)
Hokkaido University
Tel: +81-11-706-2185
Email: en-press[at]general.hokudai.ac.jp

