Controlling reactivity of polyketones by ‘masking’: development of tetraketone-based metal-ion sensor
Key points
- Complex tautomerism of polyketones was controlled by isopyrazole ‘masking’ technique
- Development of metal-ion sensor using isopyrazole-masked tetraketone
Research background
Polyketones composed of 1,3-diketone repeating units are widely known as a class of important intermediate for biological synthesis of metabolites. However, controlling their reactivity has remained difficult because of their complex tautomerism (Fig. 1).
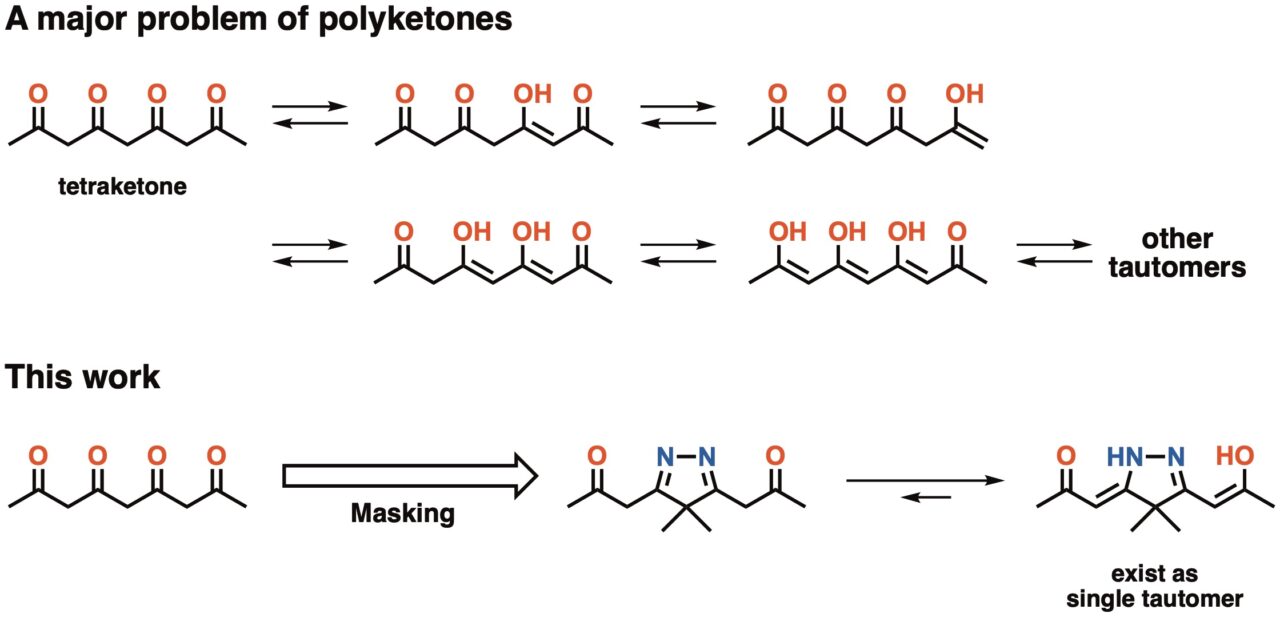 Fig. 1
Fig. 1
Results
Inokuma group in ICReDD applied isopyrazole masking technique to control tautomerism of tetraketone, an analogue of polyketones. Isopyrazole ring has two imine groups that are synthon of ketones. Synthetic conversion between interconvertible functional groups is generally called ‘masking’. When tetraketone was masked with isopyrazole, the resulting compound was found to exist as a single tautomer.
The masking technique allowed further functionalization of tetraketone to furnish metal-ion sensor (Fig. 2). The masked tetraketone-based sensor drastically changes its solid-state fluorescence emission when the solid was mixed with a solution containing metal ions such as zinc and nickel.
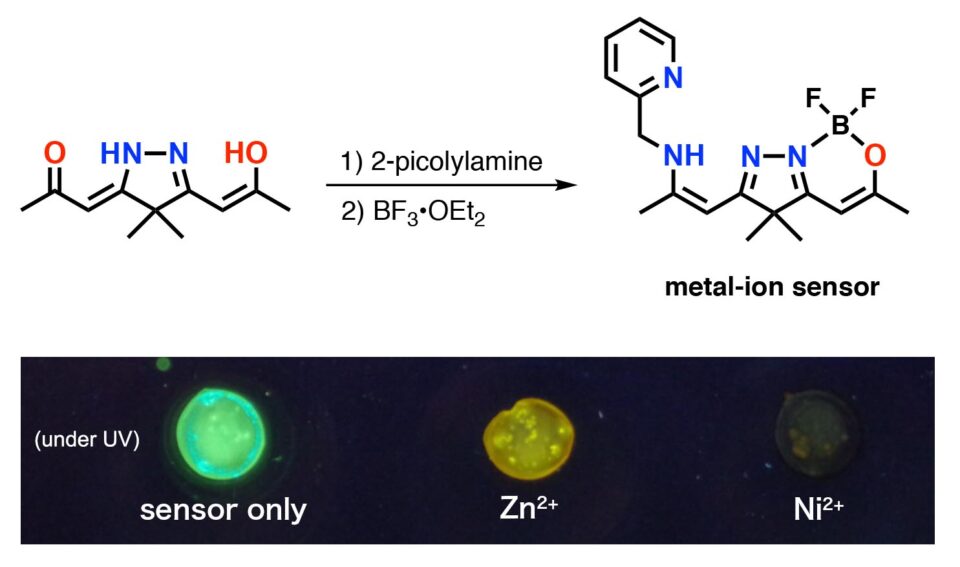 Fig. 2
Fig. 2
Future vision
The isopyrazole masking technique will widen the scope of polyketone-based chemical reactions, in particular for development of functional materials
Original paper
Isopyrazole-Masked Tetraketone: Tautomerism and Functionalization for Fluorescent Metal Ligands
Hayato Shirakura, Yumehiro Manabe, Chika Kasai, Yuya Inaba, Makoto Tsurui, Yuichi Kitagawa, Yasuchika Hasegawa, Tomoki Yoneda, Yuki Ide, Yasuhide Inokuma
DOI: 10.1002/ejoc.202100784

