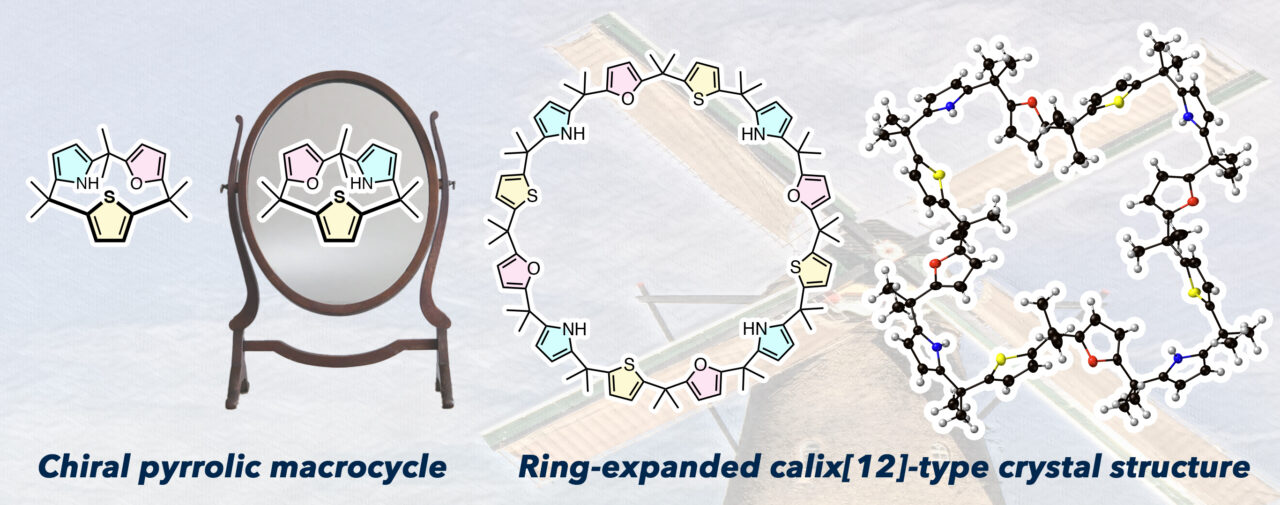
Calixpyrroles are useful in host-guest chemistry and better understanding how to control the chirality and reactivity of calix[3]pyrrole analogues could lead to their use as unique synthetic intermediates. To that end, a team of researchers from Hokkaido University, ICReDD, and the University of Texas-Austin have synthesized calix[1]furan[1]pyrrole[1]thiophene, which is a unique, chiral calix[3]pyrrole analogue that features three different subunits. The ring expansion reaction of this molecule produces 6-, 9-, and 12-membered calixpyrrole analogues. The calix[12]pyrrole analogue is the largest such analogue to ever be structurally characterized, and single crystal x-ray diffraction data showed a windmill-shaped structure.
Researchers also shed light on how to control the ring inversion behavior of the calix[1]furan[1]pyrrole[1]thiophene molecule. Despite two enantiomers of the molecule existing, simulations showed that ring inversions interconvert the molecule between these two forms, resulting in an inseparable mixture. The team found that adding a methyl group to the nitrogen atom in calix[1]furan[1]pyrrole[1]thiophene completely suppresses this mixing process and enabled the two enantiomers of the molecule to be isolated. A modest selectivity was obtained when the methyl group was added in the presence of chiral ammonium salts, showing the chirality of calix[3]pyrrole analogues can be transferred to another substrate.
This work was published as a communication in the journal Angewandte Chemie International Edition on February 13, 2023.

