Two catalysts working in tandem enable inexpensive formate salts to perform difficult dearomative reactions, giving products potentially useful for drug development.
Researchers at the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) have developed a method that uses cooperating catalysts to carry out challenging dearomative carboxylation reactions. In this process, highly reactive carbon dioxide (CO2) radical anions are derived from inexpensive formate salts and used to produce a variety of products, including α-amino acids, which are potentially useful in drug development.
Aromatic systems are stable, so carrying out a dearomative reaction, where the aromatic system is disrupted, requires a highly reactive compound. In this study, a formate salt compound is used as the source of highly reactive CO2 radical anions. Researchers designed the reaction such that two separate catalysts, a photoredox catalyst and a hydrogen atom transfer catalyst, cooperate with each other. This cooperation, combined with exposure to blue light, generates highly reactive CO2 radical anions that can add themselves to an aromatic system, disrupting the aromatic system in the process.
Previous research from this group that used electricity to drive a similar reaction provided inspiration for this study.
“Based on computations from a previous electrochemical reaction that we studied, we suspected a reaction mechanism using CO2 radical anions would be promising,” commented first author Saeesh Mangaonkar. “This time, we came up with a photochemical approach which is environmentally friendly and suitable for commercially available aromatic compounds.”
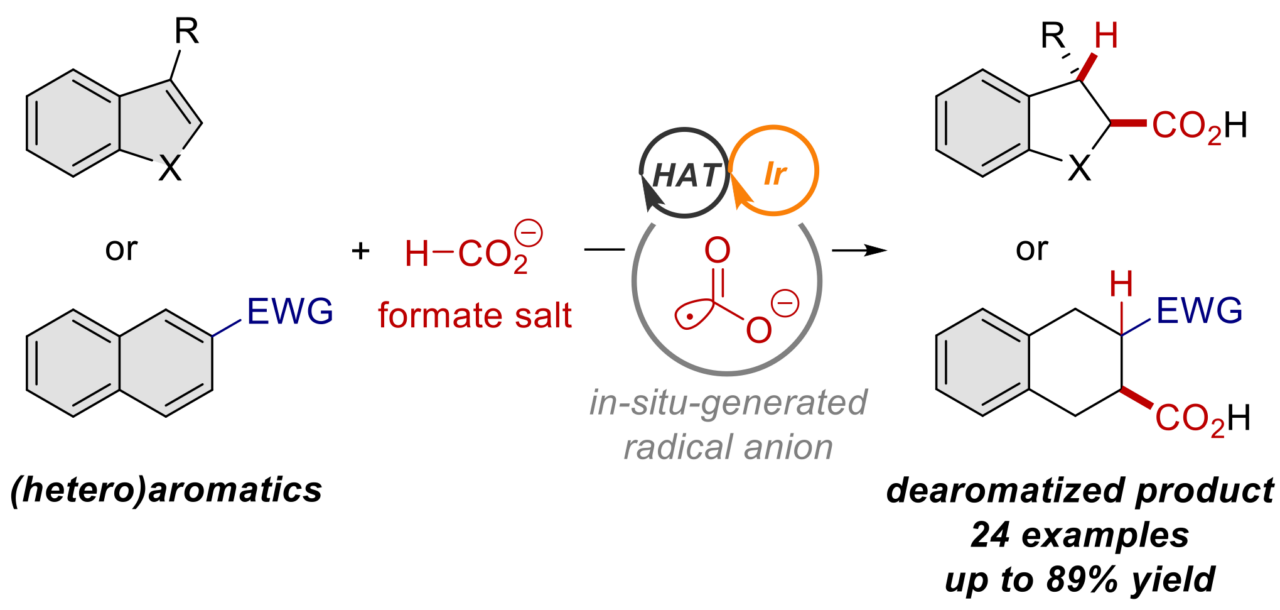
In addition to standard all-carbon aromatic rings, this dearomative carboxylation reaction framework was shown to be successful for a wide variety of heteroaromatic rings, which feature an oxygen, nitrogen or sulfur atom in the ring instead of one of the carbon atoms. The resulting carboxylic acid derivatives have non-carbon atoms neighboring the acid part of the molecule, and this is an important structural feature. With additional steps, these types of compounds can be transformed into biologically active structures, making the process developed in this study a highly useful transformation.
While the previous method adds two CO2 molecules to the substrate, this new method adds only a single CO2 molecule derived from formate salts. With these two complementary methods, chemists can now control whether they add one or two CO2 molecules, enabling greater freedom when designing and synthesizing molecules with carboxyl groups.
“We are hoping this process will prove useful in pharmaceutical development,” added Tsuyoshi Mita. “Creating efficient synthesis methods such as this one is important for working towards a sustainable society.”

Original Article:
Saeesh R. Mangaonkar, Hiroki Hayashi, Hideaki Takano, Wataru Kanna, Satoshi Maeda, Tsuyoshi Mita. Photoredox/HAT-Catalyzed Dearomative Nucleophilic Addition of the CO2Radical Anion to (Hetero)Aromatics. ACS Catalysis. February 3, 2023.
DOI: 10.1021/acscatal.2c06192
Funding:
This work was financially supported by the Japan Science and Technology Agency Exploratory Research for Advanced Technology (JST-ERATO) grant JPMJER1903, the Japan Society for the Promotion of Science (JSPS) World Premier International Research Initiative (WPI), and Grants-in-Aid for Challenging Research (Exploratory) (21K18945), Scientific Research (B) (22H02069), and Transformative Research Areas (A) [Digitalization-driven Transformative Organic Synthesis (Digi-TOS)] (22H05330), the Fugaku Trust for Medical Research, the Uehara Memorial Foundation, and the Naito Foundation.
Contacts:
Specially Appointed Associate Professor Tsuyoshi Mita
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD)
Hokkaido University
Email: tmita[at]icredd.hokudai.ac.jp
Dr. Saeesh Mangaonkar
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD)
Hokkaido University
Email: saeeshmangaonkar[at]icredd.hokudai.ac.jp
Collin Stecker (Public Relations and Outreach)
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD)
Hokkaido University
Tel: +81-11-706-9646
E-mail: public_relations[at]icredd.hokudai.ac.jp
Sohail Keegan Pinto (International Public Relations Specialist)
Public Relations Division
Hokkaido University
Tel: +81-11-706-2185
Email: en-press[at]general.hokudai.ac.jp
Related Press Releases:
CO2 recycling and efficient drug development—tackling two problems with one reaction
Chemical reaction design goes virtual
Novel computer-assisted chemical synthesis method cuts research time and cost

