A new recipe, or design guidelines, for self-strengthening muscle-like hydrogel has been developed through strategic integration of computational, information, and experimental research. The resulting gel exhibits rapid reinforcement under mechanical stress with improved stability.
Hydrogels are a permeable soft material consisting of polymer networks and water that are relevant for biological material applications. Professor Gong’s group at WPI-ICReDD has previously developed muscle-like double-network hydrogel technologies that undergo self-strengthening activated by mechanical stress. Upon mechanical stress, the brittle polymer network breaks and generates radicals that form new, stronger polymer networks by reacting with monomers pre-dispersed in the material. Recently, the group discovered that incorporating mechanophores containing weak bonds in the brittle network highly improves the efficiency of bond breaking and thereby leads to rapid self-strengthening. However, such weak bonds are also sensitive to heat and light triggered reactions, which are concerning for material stability.
For this, a team of researchers from WPI-ICReDD developed a computational design that rapidly evaluates mechanophores that contain stronger bonds but create force-sensitive polymers. Professor Maeda’s research group has been developing the Artificial Force Induced Reaction (AFIR) method that automatically explores reaction pathways. Associate Professor Jiang has developed an extended-AFIR (EX-AFIR) method that analyzes reactions induced by mechanical force and predicts the force required to break polymer chains. By combining the EX-AFIR method with machine learning, developed by Assistant Professor Staub and Professor Varnek, they rapidly screened mechanophore candidates and identified key molecular parameters. This research was published in Chemical Science on July 10, 2025.
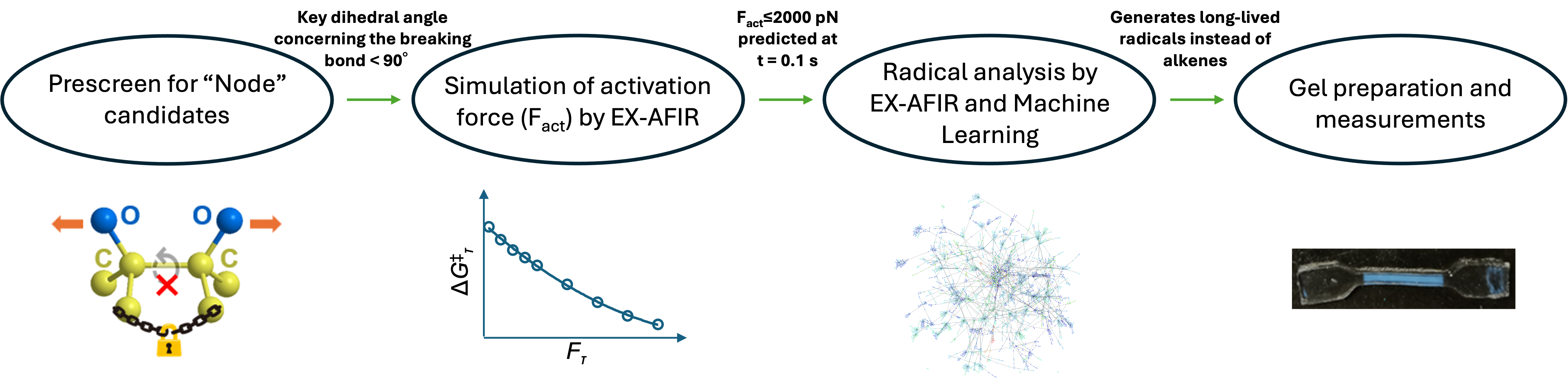
First, molecular candidates were prescreened for desirable limited rotational capability (<90°). They predicted that limited rotation within a polymer chain would effectively introduce “nodes” where the chain can break under weak force despite having strong bonds. EX-AFIR was then used to derive the activation force (Fact), the force necessary to initiate radical generation, of these candidates with a node. Lastly, EX-AFIR was combined with machine learning to calculate the decay pathways of the radicals and identify mechanophores that could generate long-lived radicals.
Gels were synthesized from selected mechanophores and investigated to confirm the practicality of this computational selection method. These gels featured rapid reinforcement and the mechanophores remained unchanged after heating at 80°C or UV light exposure for 10 hours, as expected, highlighting the significance of the node-like structure. Additionally, gels synthesized from mechanophores that were filtered out based on computational simulations were also investigated. As predicted, these gels failed to demonstrate self-strengthening qualities, further validating the practicality of this computational design approach. These results highlight the exciting opportunities of integrating computational calculations to rapidly advance technologies that would otherwise be time intensive.
The video below compares the radical generation of hydrogels suggested (DN-Cam) and filtered out (DN-Cy and DN-Pin) by computational simulations. Pre-dispersed in the gels are ferrous ions (Fe2+) and xylene orange. Radicals generated from the broken polymer networks will oxidize the ferrous ions into ferric ions (Fe2+ ® Fe3+) which then complex with xylene orange to produce a distinct orange color. As the computational simulations suggested, only the DN-Cam gel produced a noticeable color change suggesting the other gels generate unstable radicals that rapidly decay.

Original Article:
Julong Jiang, et al. “Node” Facilitated Thermostable Mechanophores for Rapid Self-strengthening in Double Network Materials. Chemical Science. July 10, 2025.
DOI: 10.1039/d5sc00151j
Funding:
SM and JJ are supported by the Japan Science and Technology Agency (JST) (ERATO grant numbers JPMJER1903 and CREST grant numbers JPMJCR19R1). ZW and JPG are supported by JSPS KAKENHI, grant number JP22H04968, JP22K21342. Support was also provided by the Institute for Chemical Reaction Design and Discovery (ICReDD), which was established by the World Premier International Research Initiative (WPI), MEXT, Japan.
Contacts:
Professor Satoshi Maeda
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD
Hokkaido University
Tel: +81-11-706-8118
Email: smaeda[at]eis.hokudai.ac.jp
Professor Jian Ping Gong
Institute for Chemical Reaction Design and Discovery (WPI-ICReDD)
Hokkaido University
Tel: +81-11-706-9011
Email: gong[at]sci.hokudai.ac.jp
Samuel Jacob (Public Relations and Outreach)
Institute for Chemical Reaction Design and Discovery
Hokkaido University
Tel: +81-11-706-9646
E-mail: public_relations[at]icredd.hokudai.ac.jp
Naoki Namba
Public Relations & Communications Division
Office of Public Relations and Social Collaboration
Hokkaido University
Tel: +81-11-706-2185
Email: en-press[at]general.hokudai.ac.jp

