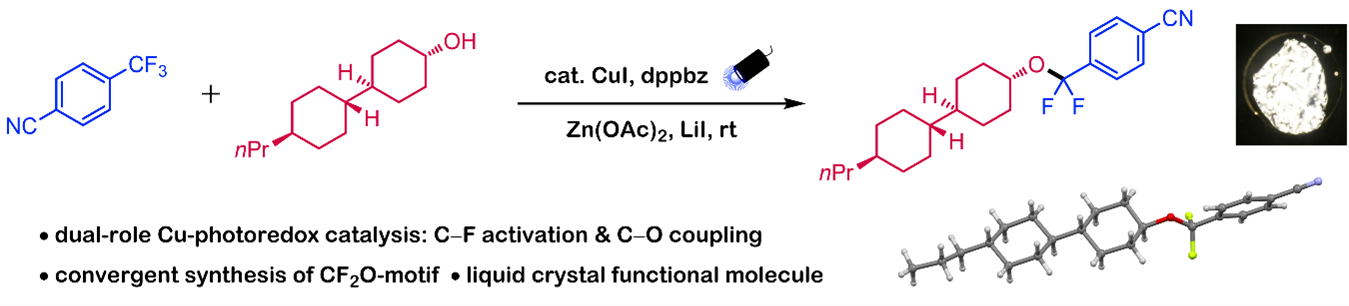
Leveraging earth-abundant and inexpensive elements, base metal catalysis has gained significant attention in recent decades for enabling new chemical reactivity. In particular, copper has emerged as a powerful candidate in photoredox catalysis due to its versatile reactivity. When paired with appropriate ligands, copper complexes can achieve sufficient reducing power under photoirradiation to activate organic substrates. A particularly intriguing class of substrates is trifluoromethyl (CF₃) compounds, which are widely found in functional molecules spanning pharmaceuticals to materials science. Selective mono-defluorinative functionalization of these motifs has the potential to expand chemical space, generating structurally unique and valuable compounds. However, existing transformations are primarily limited to C‒F to C‒H or C‒C conversions, with C‒heteroatom bond formation remaining largely unexplored.
In this work, we report a defluorinative C‒O coupling between trifluoromethylarenes (ArCF₃) and alcohols, enabled by copper photoredox catalysis. This method provides a direct and convergent approach to synthesizing difluorobenzyl ethers (ArCF₂OR) from readily available alcohols and trifluoromethylarenes. Notably, we successfully synthesized a molecule exhibiting liquid crystal behavior using this strategy. Additionally, by slightly modifying the reaction conditions, we could selectively generate difluorobenzyliodides (ArCF₂I), a class of compounds with significant potential applications.
Mechanistic investigations support a mechanism comprising dual catalytic cycle. In the C‒F activation cycle, a homoleptic copper complex ligated with two bisphosphines is proposed to act as the photocatalyst, activating ArCF₃ and generating the key ArCF₂• radical. This radical then engages in a C‒O coupling cycle mediated by another copper species bearing a single bisphosphine ligand, ultimately forming the ether product. Overall, this study demonstrates the synthesis of novel fluorinated motifs via a single copper catalytic system, which uniquely serves as both a photoredox catalyst and a cross-coupling catalyst.