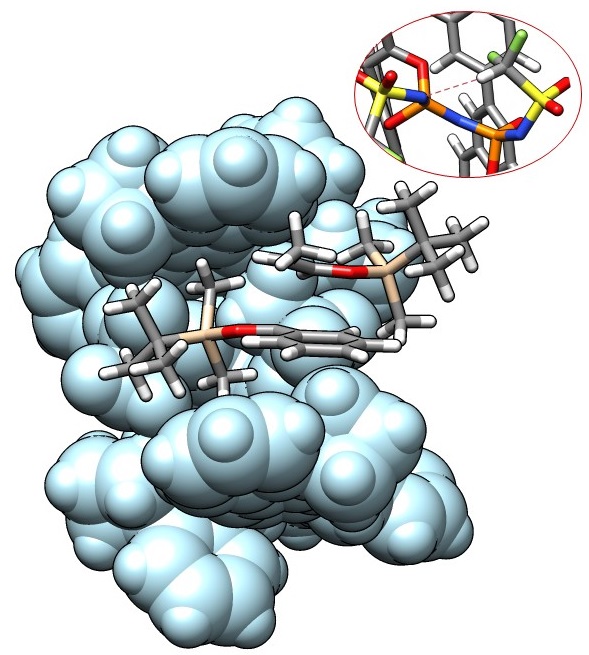
Assistant Professor and Co-PI for the List group at ICReDD Nobuya Tsuji collaborated with other members of Professor Benjamin List’s research group at the Max-Planck-Institut für Kohlenforschung to develop an asymmetric organocatalyst that enables confinement-controlled syn/anti selective Mukaiyama aldolizations of propionaldehyde enolsilanes.
The researchers found that altering the substituents on a particular area of the large catalyst scaffold resulted in a change in the size of the catalyst’s reactive “pocket”. This change in size caused the molecules in the reaction to approach the pocket from different angles, which resulted in obtaining both syn/anti selectivity and high enantioselectivity.