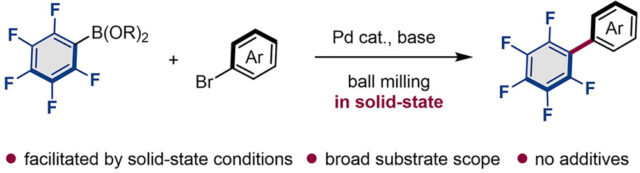
Researchers from WPI-ICReDD at Hokkaido University have discovered that the efficiency of cross-coupling reactions of weakly nucleophilic and rapidly hydrolyzable polyfluoroarylboronic acids was significantly improved under mechanochemical conditions using ball milling. This reaction proceeds in the presence of commercially available palladium-based catalysts without the use of any additives. In addition, the developed solid-state conditions also result in a reduction of solvent usage and thus provide a more environmentally friendly protocol for the preparation of polyfluorinated aromatic compounds, which are important structural motifs often found in organic functional materials.