Synthesis of Difluoroglycine Derivatives from Amines, Difluorocarbene, and CO2: Computational Design, Scope, and Applications
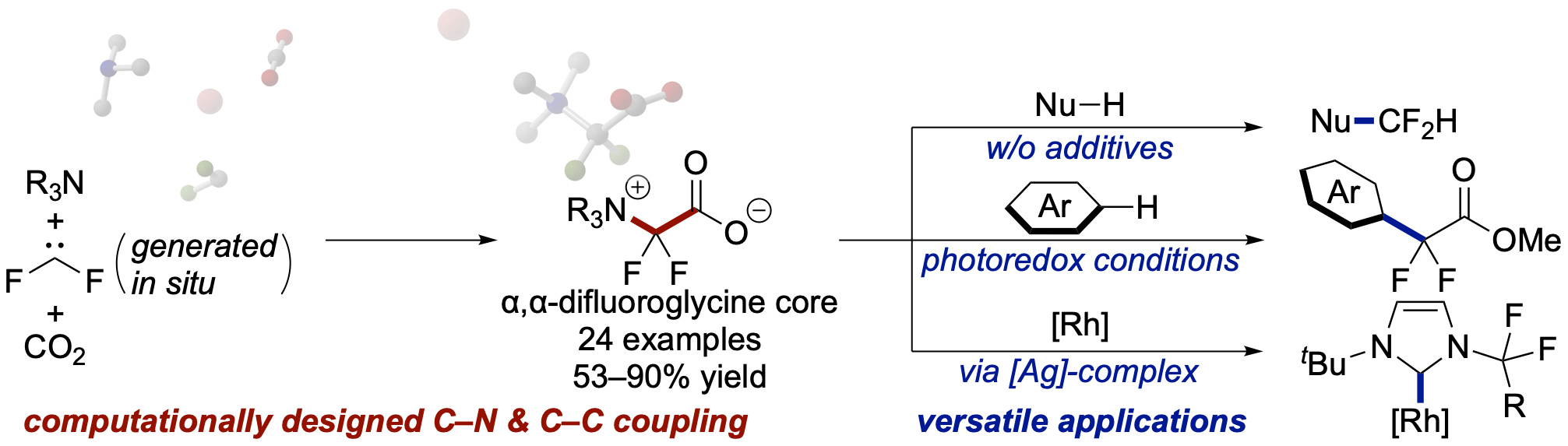
An Artificial Force Induced Reaction (AFIR) method has led to a new three-component reaction. It has enabled us to synthesis from amines, difluorocarbene, and CO2 that leads to new fluorinated glycine derivatives (α,α-difluoroglycine) with a broad substrate scope and synthetic applications. The versatility of these products is very broad; i.e., they can be used as new difluorocarbene sources, radical substitution reactions of the obtained difluoroglycine derivatives under photoredox conditions, as well as a synthetic application as an N-heterocyclic carbene ligand. More information can be found in the Full Paper by S. Maeda, T. Mita et al. (DOI: 10.1002/chem.202100812).
Original paper
Synthesis of Difluoroglycine Derivatives from Amines, Difluorocarbene, and CO2: Computational Design, Scope, and Applications
Hiroki Hayashi, Hideaki Takano, Hitomi Katsuyama, Yu Harabuchi, Satoshi Maeda, Tsuyoshi Mita
Chemistry A European Journal
DOI: 10.1002/chem.202100812
Link to Cover Picture
Link to Cover Profile

